The Cajal body (CB), one of the biological condensates in the nucleus, was identified over 100 years ago and is involved in forming ribonucleoproteins (RNPs), such as snRNPs/snoRNPs. In addition, CBs play an essential role in nonsense-mediated mRNA decay, RNAi-based gene silencing, viral infection, and stress response. Several human diseases are associated with mutations in genes encoding CB components. Therefore, CBs are nuclear structures essential for various nuclear transactions in eukaryotes. One of the most representative constituents of CBs is Coilin, an approximately 80 kDa nuclear protein conserved in multicellular organisms, from Trichoplax adhaerens to humans. Coilin is essential for the integrity of CBs. However, how Coilin regulates the formation of CBs and its functions remain elusive. As Coilin has not been identified in lower eukaryotic model organisms like C. elegans and yeast, characterizing Coilin in these organisms would be a critical step forward in better understanding Coilin.
Recently, the team led by Assistant Professor Tomoyasu Sugiyama at the School of Life Science and Technology (SLST), ShanghaiTech University, has identified the ortholog of Coilin, Mug174, an integral constituent of CBs, in the fission yeast Schizosaccharomyces pombe (S. pombe) and has unveiled novel roles of Coilin/CBs in G0/cellular quiescence. The study was published in a paper titled “The fission yeast ortholog of Coilin, Mug174, forms Cajal body-like nuclear condensates and is essential for cellular quiescence,” in the journal Nucleic Acids Research.

In the comparative analysis, the researchers found that Mug174 displays characteristics of Coilin: (1) Mug174 has an intrinsically disordered domain and can form phase-separated biomolecular condensates; (2) Mug174 forms nuclear foci that overlap with the nucleolus and the cleavage body; (3) Mug174 interacts with the trimethylguanosine (TMG) synthase Tgs1 and U snRNAs; and (4) Mug174 is required for TMG capping of U snRNAs and mRNA splicing. These findings indicated that Mug174 is the fission yeast ortholog of Coilin and that fission yeast also possesses Cajal body-like nuclear condensates.
Critically, Mug174 is indispensable for maintaining and transitioning from cellular quiescence, a resting state in which cells do not divide but retain the capacity to revert to the cell cycle in response to appropriate stimuli. In this regard, Mug174 exerts its function in G0 by facilitating mRNA splicing and preventing RNA polymerase I transcription.
In conclusion, the identification of the first Coilin ortholog in unicellular organisms marks a significant milestone in understanding the evolutionary origins of Coilin/CBs in eukaryotes. It also provides critical insights into the regulatory mechanisms of Coilin/CBs in G0 maintenance/exit, offering a key explanation for the association between Cajal body dysfunction and human diseases, such as neurodegenerative diseases. This work not only deepens the knowledge of cellular quiescence but also paves the way for future studies in the nuclear body/cellular quiescence fields.
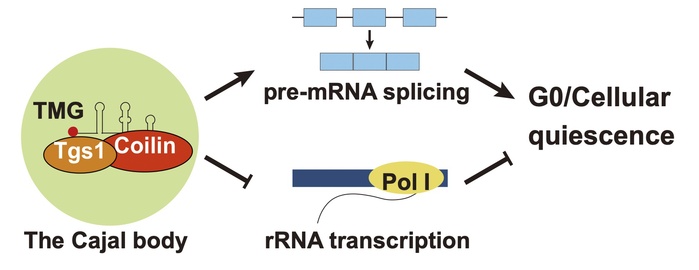
Regulatory mechanisms of Coilin in cellular quiescence
Deng Xiaoling, a PhD student in SLST, is the first author of the paper, and Prof. Tomoyasu Sugiyama is the corresponding author.
*This article is provided by Prof. Tomoyasu Sugiyama

